Technical Articles

Pharmaceutical Stability Test Chamber Application and 2025 Edition Pharmacopoeia Standard In-depth Analysis
Pharmaceutical Stability Test ChamberAs the core equipment for quality control in the pharmaceutical industry, its importance has become more and more prominent with the implementation of the 2025 edition of the Chinese Pharmacopoeia. In this paper, we will systematically analyze the latest requirements and practices of drug stability research from four dimensions, namely, equipment application, new regulation changes, testing methods and technology development.
First, the core function of the drug stability test chamber and industry applications
1.1 Precision Control System Component Technology Cornerstone
Modern pharmaceutical stability test chambers use advanced PID (Proportional-Integral-Differential) control algorithms with high sensitivity platinum resistance temperature sensors and capacitive humidity sensors, capable of achieving a temperature control accuracy of ±0.3°C and a humidity fluctuation range of ±2%RH. This level of control is critical for obtaining reliable stability data, especially for special pharmaceuticals such as vaccines and biologicals that are sensitive to temperature.
The air circulation system adopts three-dimensional three-dimensional air supply technology, through the computational fluid dynamics (CFD) optimized air duct design, to ensure that the temperature and humidity differences between the various points in the box does not exceed ± 1 ℃ and ± 3% RH. Comparative tests of an international pharmaceutical company showed that, compared with the traditional test chamber, three-dimensional three-dimensional air supply technology of the equipment can make the difference between the samples to reduce the difference of 42%, which significantly improves the consistency of the data.
1.2 Multi-functional applications covering the full life cycle of medicines
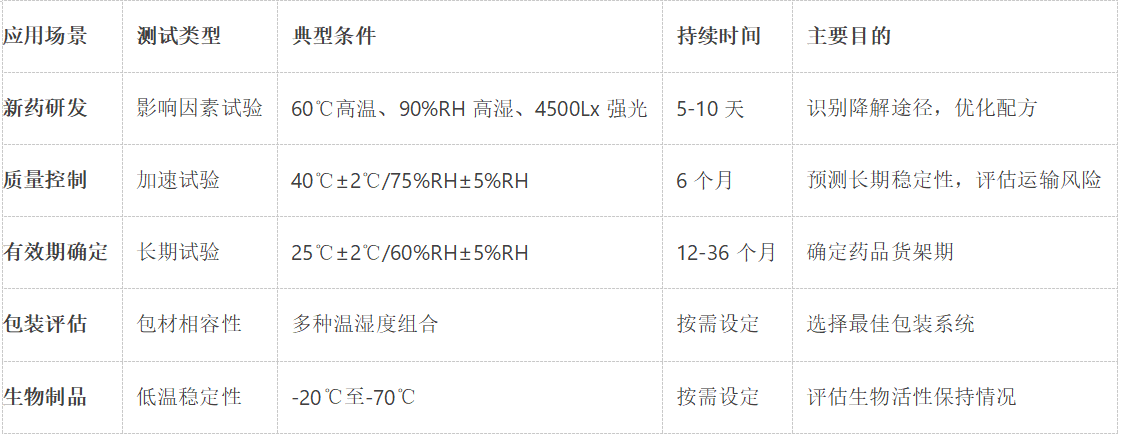
Stability test chambers are mainly used for forced degradation tests during the development phase of new drugs. Typical test conditions according to ICH Q1A guidelines include:
High temperature test: 10 days at 60℃.
High humidity test: 25℃/90%RH environment exposure for 10 days
Light test: 4500Lx±500Lx illumination test 10 days
The case of a domestic innovative pharmaceutical company shows that, through systematic forced degradation tests, its antitumor drug under development identified photosensitivity problems at an early stage, which led to timely adjustment of the packaging scheme and avoided subsequent development risks.
In the commercial production phase, the stability test chamber mainly undertakes three key tasks:
Accelerated stability test: 6 months test under 40℃±2℃/75%RH±5%RH conditions
Long-term stability test: 25℃±2℃/60%RH±5%RH for 12-36 months.
Intermediate condition test: 30℃±2℃/65%RH±5%RH as supplementary test
Second, the 2025 edition of the Pharmacopoeia on the stability test of the key revisions and response strategy
2.1 Significant updates to the testing system for pharmaceutical packages
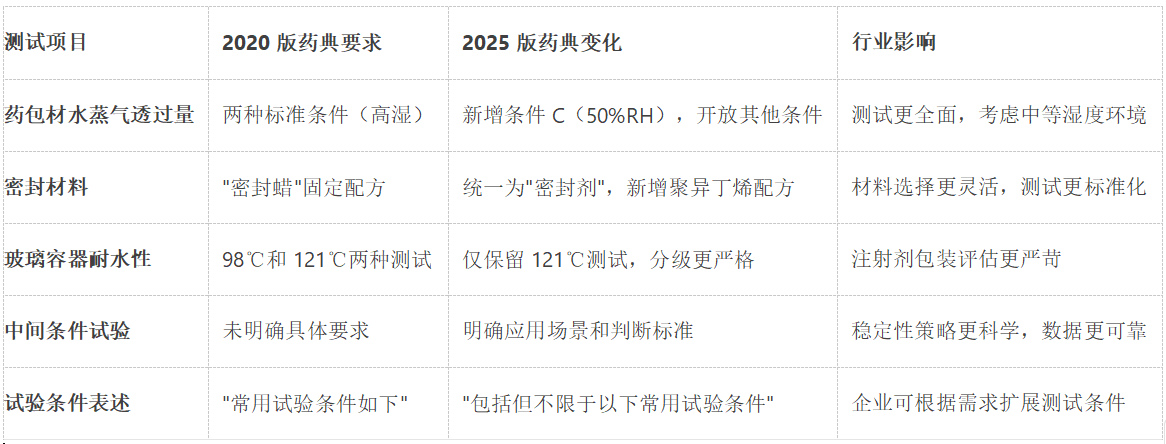
The 2025 edition of the Pharmacopoeia contains a comprehensive revision of the method for determining the water vapor transmission rate of pharmaceutical packages (General Rule 4010), and the main changes include:
New test conditions C: temperature 23 ℃ ± 2 ℃, relative humidity 50% ± 5%, filling the gap of medium humidity test
Expanding the expression of test conditions: amending "commonly used test conditions are as follows" to "including but not limited to the following commonly used test conditions", which provides a regulatory basis for testing under special climatic conditions.
Diversification of desiccant selection: on the basis of retaining anhydrous calcium chloride, new silica gel, molecular sieve and other options, requiring effective activation before use.
Test data from a multinational pharmaceutical packaging materials company showed that at 23°C/50%RH, the moisture permeability of certain composite films differed significantly from that at 40°C/90%RH (with an average difference of 35%), highlighting the practical value of the new test conditions.
2.2 Significant improvement in packaging standards for injectables
The 2025 edition of the Pharmacopoeia makes important adjustments to water resistance testing of glass containers for injectables:
Eliminate the 98°C test and retain only the 121°C autoclave test requirement
Strict grading criteria, deletion of non-pharmaceutical related level 3 water resistance requirement
New surface water resistance test for more comprehensive evaluation of glass container performance
These changes further align the Chinese standard with the European Pharmacopoeia (EP) and the United States Pharmacopoeia (USP). According to the National Testing Center for Pharmaceutical Packaging Materials (NTPCM), after the implementation of the new standards, the qualification rate of domestically produced neutral glass is expected to drop from 92% to 87%, prompting companies to upgrade their production processes.
2.3 Clear specification of intermediate condition tests
The 2025 edition of the Pharmacopoeia specifies in detail for the first time the application scenarios of the intermediate conditions test (30°C ± 2°C/65%RH ± 5%RH):
To be activated when there is a "significant change" in the acceleration test.
Clarify the criteria for determining "significant change":
Content ≥5% from initial value
Any degradation products exceeding standard limits
pH out of range
Dissolution non-compliance (1 out of 12 tablets exceeded the limit)
The actual case of a generic drug enterprise shows that a 4.8% decrease in content was found at 3 months of accelerated testing, and the change was confirmed to be due to the variation of analytical method after the intermediate condition test was immediately initiated, avoiding unnecessary product scrapping.
III. Differentiated testing strategies and validity period determination methods
3.1 Scientific establishment of classification testing strategies
Stability assessment of chemicals:
Impact factor test: 60℃/75%RH/4500Lx conditions
Items to focus on: substances of interest (with special attention to degradation products), content, solubility
Packaging options: moisture resistance level based on high humidity test results
Stability data for an antibiotic product showed that the degradation rate at high humidity conditions (90% RH) was 2.3 times higher than that of aluminum foil laminate packaging under aluminum blister packaging, which directly affected the final packaging decision.
Special requirements for biological products:
Storage conditions: usually 2-8℃, some varieties need -20℃ or -70℃.
Key indicators: biological activity, polymer content, visible foreign matter
Freeze-thaw test: at least 3 cycles from -20℃ to 25℃.
A stability study of a monoclonal antibody drug showed that after three freeze-thaw cycles, its aggregates content increased from 0.5% to 1.8%, prompting the company to optimize its cold chain transportation scheme.
3.2 Scientific methodology for determining the period of validity
The determination of a drug's expiration date requires a combination of data:
Long-term test data: primary basis, usually covering the proposed validity period
Accelerated trial data: supporting evidence for extrapolation
Regular drugs: 6 months accelerated data can support 24 months expiration date
Refrigerated medicines: extrapolate no more than the time of the long-term test plus 6 months
Statistical analysis methods:
Analyzing degradation trends using linear regression
Calculation of 95% unilateral confidence limits and quality standard intersections
Consideration of inter-batch variability (typically ≥3 batches of data)
A case study on the stability analysis of a hypoglycemic tablet showed that the expiration date predicted by statistical modeling was 32 months, and was ultimately conservatively determined to be 24 months, reflecting a balance of science and risk control.
IV. Technological Innovation and Industry Best Practices
4.1 Key technological advances
Temperature and humidity control technology:
Adaptive PID control: automatically adjusts parameters according to load changes
Dual refrigeration systems: ensuring continuous operational reliability
Wireless sensor network: real-time monitoring of environmental parameters at multiple points in the box
A brand of test chamber test data show that the use of adaptive PID control, temperature fluctuations from ± 0.5 ℃ to ± 0.2 ℃, energy consumption at the same time reduced by 15%.
Data management systems:
Compliance with 21 CFR Part 11: full audit trail, electronic signatures
Cloud Data Backup: Preventing Data Loss
Intelligent Early Warning System: Predicting Equipment Failures Based on Machine Learning
4.2 Verification and calibration systems
Sound equipment management should include:
IQ/OQ/PQ validation (3Q validation):
Installation Qualification (IQ): Verify that the equipment is installed according to specifications
Operational confirmation (OQ): test that the functions are working correctly
Performance Qualification (PQ): Verification of temperature and humidity performance under actual loads
Periodic calibration:
Temperature sensors: calibrated every 6 months
Humidity sensor: calibrated every 12 months
Use of NIST-traceable standardizers
The experience of a GMP-certified company showed that after implementing a rigorous calibration program, the anomaly rate of stability data was reduced from 3.2% to 0.7%.
4.3 Continuous improvement trends
The industry is moving in the following direction:
Modular design: flexible configuration of temperature and humidity ranges, light modules, etc.
Green energy saving: inverter compressor, heat recovery system
Intelligent management: remote monitoring, predictive maintenance
Data integration: seamless integration with LIMS (Laboratory Information Management System)
The implementation of the 2025 edition of the Pharmacopoeia will drive drug stability research into a new phase. Pharmaceutical companies need to fully understand the regulatory changes, select technologically advanced test equipment and establish scientific testing programs in order to ensure the stability of the quality of drugs throughout their life cycle. This is not only related to the compliance of enterprises, but also an important guarantee for the safety of patients' medication.

 +86 13917986725
+86 13917986725